# References & Parameters
Note
This collection of references and equations is in no way complete nor is it intended to be. It is just a guideline to get you started in case you are working on your own calculations. If you use the equations, make sure to cite the publications accordingly.
# Reviews & Articles
For a general purpose overview of fluorescence- and absorbance-based photosynthetic parameters, and their interpretation, we recommend the following sources:
Kramer, D.M., Cruz, J.A. and Kanazawa, A. (2003) Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci. 8: 27-32 doi:10.1016/S1360-1385(02)00010-9 (opens new window)
Baker, N.R., Harbinson, J., and Kramer, D.M. (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 30: 1107-1125 doi:10.1111/j.1365-3040.2007.01680.x (opens new window)
Baker, N.R. (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 59: 89-113 doi:10.1146/annurev.arplant.59.032607.092759 (opens new window)
Kramer, D. M., and Evans, J. R. (2011). The importance of energy balance in improving photosynthetic productivity. Plant Physiol. 155, 70–8. doi:10.1104/pp.110.166652 (opens new window).
Kalaji, H. M., Schansker, G., Ladle, R. J., Goltsev, V., Bosa, K., Allakhverdiev, S. I., et al. (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynthesis Research 122, 121–158.doi:10.1007/s11120-014-0024-6 (opens new window).
Cruz, J. A., Savage, L. J., Zegarac, R., Hall, C. C., Satoh-Cruz, M., Davis, G. A., et al. (2016). Dynamic Environmental Photosynthetic Imaging Reveals Emergent Phenotypes. Cell Syst. 2, 365–377. doi:10.1016/j.cels.2016.06.001 (opens new window).
Kalaji, H. M., Schansker, G., Brestic, M., Bussotti, F., Calatayud, A., Ferroni, L., et al. (2016) Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynthesis Research 132, 13–66. doi:10.1007/s11120-016-0318-y (opens new window).
Cruz, J. A. & Avenson, T. J. (2021) Photosynthesis: a multiscopic view. J Plant Res 134, 665–682. doi:10.1007/s10265-021-01321-4 (opens new window).
# Environmental Parameters
# Light
| Parameter | Details |
|---|---|
| Light Intensity (PAR) | Photosynthetically active radiation. Fraction of the incoming light (400 - 700 nm) which can be utilized for photosynthesis; µmol photons × s⁻¹ × m⁻² |
| R | The raw amount of red light captured by the PAR sensor |
| G | The raw amount of green light captured by the PAR sensor |
| B | The raw amount of blue light captured by the PAR sensor |
# Atmospheric Parameters
| Parameter | Details |
|---|---|
| Ambient Humidity | Relative humidity in percent (%) |
| Ambient Pressure | Atmospheric pressure (mbar) - This value is not corrected to sea level as found in weather reports |
| Ambient Temperature | Ambient temperature in degree Celsius (℃) |
# Leaf Parameters
| Parameter | Details |
|---|---|
| contactless_temp | Surface temperature in degree Celsius (℃) |
| Leaf Temperature Differential | Leaf Temperature ( or contactless_temp) minus Ambient Temperature, negative numbers mean that the leaf is cooler than the surrounding air and vice versa. |
| Thickness | The thickness of the leaf as measured by the Hall Effect sensor in (µm) |
# Positional Information
| Parameter | Details |
|---|---|
| angle_direction, compass_direction | Abbreviated cardinal direction (e.g. NW - North West) |
| compass | Cardinal direction in degrees from North |
| Leaf Angle | The angle of the leaf, from 0 - 90 degrees |
| roll | Roll is the angle the Instrument is held along the long axis |
| pitch | Pitch is the angle the Instrument is held along the short axis |
# Chlorophyll Fluorescence (PAM)
Here you find commonly used equations to derive photosynthetic parameters from fluorescence changes measured using the Pulse-Amplitude-Modulation (PAM) method. Please be aware, that depending on the literature, the same parameters can have multiple names.
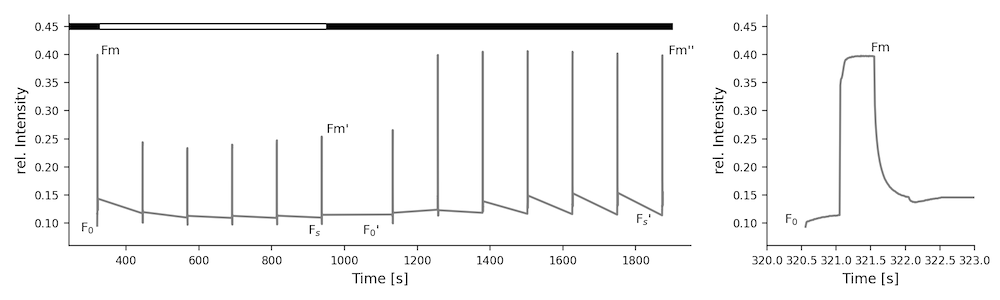
# Yield and Electron Transfer
# Maximum Quantum Efficiency
(1)
# Quantum Yield
(2)
- Genty, B., Briantais, J.-M. & Baker, N.R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence Biochimica et Biophysica Acta (BBA) - General Subjects 990(1), pp.87–92. doi:10.1016/S0304-4165(89)80016-9 (opens new window).
# Linear Electron Flow
(3)
# Non-regulatory Energy Dissipation
(4)
- Kuhlgert, S., Austic, G., Zegarac, R. Osei-Bonsu, I.,Hoh, D., Chilvers, M. I., et al. (2016). MultispeQ Beta: a tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 3, 160592. doi:10.1098/rsos.160592 (opens new window).
# Non photochemical quenching (NPQ)
(7)
- Genty, B., Briantais, J.-M. & Baker, N.R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence Biochimica et Biophysica Acta (BBA) - General Subjects 990(1), pp.87–92. doi:10.1016/S0304-4165(89)80016-9 (opens new window).
(8)
- Kuhlgert, S., Austic, G., Zegarac, R. Osei-Bonsu, I.,Hoh, D., Chilvers, M. I., et al. (2016). MultispeQ Beta: a tool for large-scale plant phenotyping connected to the open PhotosynQ network. R. Soc. Open Sci. 3, 160592. doi:10.1098/rsos.160592 (opens new window).
(9)
- Tietz, S., Hall, C. C., Cruz, J. A., Kramer, D. M. (2017) NPQ(T): a chlorophyll fluorescence parameter for rapid estimation and imaging of non-photochemical quenching of excitons in photosystem-II-associated antenna complexes Plant. Cell Environ. 40(8), 1243–1255. doi:10.1111/pce.12924 (opens new window).
# Energy-Dependent Quenching
(10)
- Weis, E., and Berry, J. A. (1987) Quantum efficiency of Photosystem II in relation to “energy”-dependent quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA) - Bioenergetics 894, 198–208.
# Stern-Volmer-coefficients of qE
(11)
- Doege, M., Ohmann, E. & Tschiersch, H. (2000). Chlorophyll fluorescence quenching in the alga Euglena gracilis. Photosynthesis Research 63, 159–170. doi:10.1023/A:1006356421477 (opens new window)
# Photo-Inhibitory Quenching
(12)
- Quick, W. P., and Stitt, M. (1989). An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochimica et Biophysica Acta (BBA) - Bioenergetics 977, 287–296. doi:10.1016/S0005-2728(89)80082-9 (opens new window)
# Photosystem II Redox State
# "Puddle" model
(5)
- Krause, G. H., Vernotte, C., and Briantais, J.-M. (1982) Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae. Resolution into two components. Biochimica et Biophysica Acta (BBA) - Bioenergetics 679, 116–124. doi:10.1016/0005-2728(82)90262-6 (opens new window)
# "Lake" model
(6)
- Kramer, D.M., Johnson, G., Kiirats, O., and Edwards, G.E. (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 79: 209-218 doi:10.1023/B:PRES.0000015391.99477.0d (opens new window)
# Electrochromic Shift (ECS)
These are commonly used equations to derive ATP-Synthase activity by measuring the electrochromic shift using the Dark Interval Relaxation Kinetic (DIRK) method. The ECS signal at a given time after the start of the DIRK dark interval, ECSt is the maximum amplitude of the ECS DIRK signal, related to the light-dark difference in thylakoid pmf. Tau (τ) is the relaxation time for the ECS signal, which is inversely related to the activity of the ATP synthase. Note that for this fitting to work, the baseline for the ECS signal is taken to be the points just prior to the dark interval so that the ECS decay in the dark results in a negative signal.
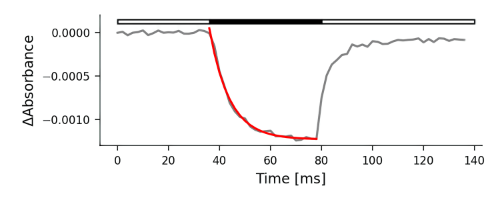
# ECSt - Magnitude of Electrochromic Shift
(1)
- Sacksteder, C. A., Kanazawa, A., Jacoby, M. E., Kramer, D. M. (2000). The proton to electron stoichiometry of steady-state photosynthesis in living plants: A proton-pumping Q cycle is continuously engaged. Proc. Nat. Acad. Sci. 97, 14283-14288. doi:10.1073/pnas.97.26.14283 (opens new window)
- Kramer, D. M., Sacksteder, C. A., Cruz, J. A. (1999). How acidic is the lumen? Photosynthesis Res. 60: 151-163 doi:10.1023/A:1006212014787 (opens new window)
- Cruz, J.A., Sacksteder, C.A., Kanazawa, A., and Kramer, D.M. (2001) Contribution of electric field (delta psi) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into delta psi and delta pH by ionic strength. Biochemistry 40: 1226-1237 doi:10.1021/bi0018741 (opens new window)
# gH⁺ - Proton conductivity
(2)
- Kanazawa, A., Kramer, D. K. (2002). In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Nat. Acad. Sci. 99: 12789-12794. doi:10.1073/pnas.182427499 (opens new window)
# vH⁺ - Steady-State Proton Flux
(3)
- Avenson, T. J., Kanazawa, A., Cruz, J. A., Takizawa, K., Ettinger, W.E., Kramer, D. K. (2005). Integrating the proton circuit into photosynthesis: progress and challenges. Plant Cell Environ. 28: 97-109. doi:10.1111/j.1365-3040.2005.01294.x (opens new window).
# Photosystem I Redox State
- Harbinson, J., and Hedley, C.L., (1989) The kinetics of P-700+ reduction in leaves: a novel in situ probe of thylakoid functioning. Plant, Cell. Environ. 12: 357-369 doi:10.1111/j.1365-3040.1989.tb01952.x (opens new window)
- Kanazawa, A., Ostendorf, E., Kohzuma, K., Hoh, D., Strand, D. D., Sato-Cruz, M., Savage, L., Cruz, J. A., Fisher, N., Froehlich, J. E., Kramer, D. K. (2017). Chloroplast ATP Synthase Modulation of the Thylakoid Proton Motive Force: Implications for Photosystem I and Photosystem II Photoprotection. Front. Plant Sci. 8:1-12. doi:10.3389/fpls.2017.00719 (opens new window).
# Relative Chlorophyll
# SPAD - Special Products Analysis Division
Calculate the leaf's relative chlorophyll content from measuring the absorbance at 650 nm and 940 nm.
(1)
(2)
is the arbitrary (and proprietary) correlation coefficient used in the Minolta SPAD, but approximated using the MultispeQ calibration cards.
- Maas, S.J. & Dunlap, J.R. (1989). Reflectance, Transmittance, and Absorptance of Light by Normal, Etiolated, and Albino Corn Leaves. Agronomy Journal, 81(1), p.105. doi:10.2134/agronj1989.00021962008100010019x (opens new window)
- Raymond Hunt, E., and Daughtry, C. S. T. (2014) Chlorophyll Meter Calibrations for Chlorophyll Content Using Measured and Simulated Leaf Transmittances. Agronomy Journal 106, 931–939. doi:10.2134/agronj13.0322 (opens new window)
# Links
If you want to add a link to the platform, please use: https://photosynq.org (opens new window)
Also we would like to strongly encourage you to share the link(s) to your projects so others can see the data you have collected. This goes hand in hand with writing a short result section on the platform, perhaps including a link to your publication...
Parameter Availability & Calculation
The availability of parameters and how they are derived depends on the version and type of the Instrument, as well as on the Measurement Protocol. Please take that into consideration when analyzing your data.